MODERNE UND EFFIZIENTE ANALYTIK
Mikrobiologische Untersuchungen führen wir in eigenen hochmodernen Einrichtungen durch, chemische und physikalische Tests bieten wir in Zusammenarbeit mit kompetenten Partner-Laboratorien an.
Mit einem breiten Spektrum an Labordienstleistungen integriert sich GfPS nahtlos in alle Ihre Forschungs- und Entwicklungsprojekte. Neben Testungen der normativ geregelten Parameter erstellen wir für Ihre speziellen Fragestellungen individuelle Lösungskonzepte. Zusätzlich zu Laboranalytik und Messtechnik gehört die kompetente Beantwortung Ihrer hygienerelevanten Fragen zu unseren Schwerpunkten:
MIKROBIOLOGISCHE ANALYTIK
HYGIENEBETREUUNG
SCHULUNGEN
AUDITS

SERVICES
Pharmaceutical and medical products: Production conditions
- Prüfung der Reinräume / raumlufttechnischen Anlagen auf fortlaufende Übereinstimmung mit DIN EN ISO 14644, DIN EN 17141 und dem Leitfaden für die gute Herstellungspraxis (EU GMP)
- Hygiene-Prüfung der Produktionsbedingungen und Ermittlung des Hygienestatus
- Quantitative Ermittlung der Bioburden gemäß DIN EN ISO 11737-1
- Prüfung auf bakterielle Endotoxine (LAL-Test), Festgel und kinetisch-turbidimetrische Methode (Ph. Eur., USP)
- Mikrobiologische Identifizierungen
- Molekularbiologische Identifizierung von Mikroorganismen
- mehr
Pharma- und Medizinprodukte: Überwachung / Validieren von Sterilisationsprozessen
- Überwachung und Validierung von Sterilisationsprozessen mittels Bioindikatoren
- Prüfungen auf Sterilität gemäß Ph. Eur., USP
- Prüfung der Sterilität gemäß DIN EN ISO 11737-2
- Validierung, Revalidierung und Leistungsbeurteilung von Sterilisationsprozessen (Strahlen- und ETO-Sterilisation)
- Mikrobiologische Prüfung von Sterilisatoren mittels Bioindikatoren
Pharmaceutical and medical products/devices: Testing according to European Pharmacopoeia / USP / other standards
- Nachweis der Abwesenheit bestimmter Mikroorganismen
- Zählung der gesamten vermehrungsfähigen aeroben Keime (TAMC, TYMC)
- Prüfung auf bakterielle Endotoxine (LAL-Test)
- Prüfung auf Sterilität
- Testung der antibakteriellen Wirksamkeit von Oberflächen und festen Körpern gemäß JIS Z 2801, ISO 22196
- Agardiffusionstest gemäß Ph. Eur,
Verpackungsprüfungen für Medizin- und Pharmaprodukte
- Verpackungsprüfungen nach DIN EN ISO 11607-1
- Sichtkontrolle (ASTM F1886 / F1886M)
- Beschleunigte Alterung (ASTM F1980)
- Echtzeitalterung (ASTM F1980)
- Transportsimulation (ASTM D4169)
- Bubble-Test (ASTM F2096)
- Microbiological Dusting (zur Prüfung der mikrobiellen Barriere)
- Peel Test (DIN EN 868, Teil 5)
- Bestimmung der Siegelnahtbreite (DIN EN 868, Teil 5)
- Zugfestigkeitsprüfung der Siegelnähte (DIN EN 868, Teil 5, ASTM F88/ F88M)
- Prüfung auf Undurchlässigkeit und Kontinuität der Siegelung (ASTM F1929, ASTM F3039)
- Prüfung auf feine Löcher in der Kunststoff-Verbundfolie (DIN EN 868, Teil 5)
- Prüfung der Keimdichtigkeit DIN 58953-6
- mehr
Hospitality and medical practice hygiene
- Prüfung von raumlufttechnischen Anlagen gemäß DIN 1946 einschließlich mikrobiologischer Umgebungsuntersuchungen und Begutachtung
- Mikrobiologische Prüfung von Sterilisatoren
Laundry / textiles
- Microbiological water tests to determine the hygienic status
- Determination of microbial contamination (bioburden) of textiles with an exceptionally high requirement for low microbial count
- Microbiological testing of sterilisers using biological indicators
Environment
- Schimmelpilzdifferenzierungen und Identifizierungen
- Antimikrobielle Wirksamkeitstests gemäß JIS Z 2801, ISO 22196
PHARMAZEUTIKA
Die Pharmaindustrie hat traditionell besonders hohe Qualitätsansprüche. Wir unterstützen unsere Kunden aus der Pharmabranche zuverlässig bei der Umsetzung dieser Ziele.
Die GfPS ist GMP-zertifiziert. – Das GMP-Dokument bescheinigt, dass wir als pharmazeutisches Prüf-laboratorium im Sinne des § 14 (4) des Arzneimittelgesetzes (AMG) mikrobiologische Qualitätskontrolluntersuchungen, insbesondere Sterilitätsprüfungen und Endotoxintests, durchführen und dabei die Anforderungen des EG-Leitfadens einer Guten Herstellungspraxis für Arzneimittel grundsätzlich erfüllen.
MEDIZINPRODUKTE
Bei Medizinprodukten werden ähnlich hohe Anforderungen gestellt wie bei Pharmazeutika. Wir unterstützen Sie sowohl bei der Produktanalytik als auch bei der Planung, Prüfung und Optimierung Ihrer Produktionsbedingungen.
Die Palette der Medizinprodukte ist enorm vielfältig und reicht von Kontaktlinsen-Pflegemitteln über Verbandmittel und Dialysegeräte bis hin zu Kathetern.
Durch die Akkreditierung bei der DaKKs nach DIN EN ISO/IEC 17025, Richtlinien 93/42/EWG und 90/385/EWG in den Bereichen mikrobiologisch-hygienische Prüfungen von Medizinprodukten einschließlich Sterilisationsverfahren und Umgebungsüberwachungen werden die von der GfPS in diesem Bereich durchgeführten Untersuchungen europaweit zur Konformitätsbewertung anerkannt.

HOSPITAL / MEDICAL PRACTICE HYGIENE
In the area of hygiene in hospitals and medical practices GfPS mbH provides monitoring of environmental conditions. Additionally, the equipment for sterilisation can also be inspected. From the shipment of biological indicators and advice on local use to the evaluation of the appropriate test systems – GfPS is at your side to advise you.

LAUNDRY
Our analytical services include efficacy studies of detergents and cleaning products for various uses (clinical, food, industrial, domestic, public facilities, etc.).

TEXTILES
Modern functional textiles must deliver maximum performance. We check the effectiveness of your products and determine the hygiene-relevant parameters. With modern textile analysis, we support you with the quality assurance of your production, e. g. the determination of microbial contamination (bioburden) of textiles with an exceptionally high requirement for low microbial count
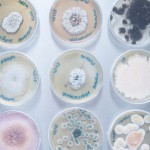
ENVIRONMENT
There are several aspects of environmental analysis that are generally summarised in the term “environment”. These include the analysis of water samples as well as the identification of species in the domain of mould analysis. Our years of experience and the annual training of our professionals in this area ensure our continued expertise and the high quality of your analysis results.
